I found this post hard to understand but here is my attempt at a summary:
This is far outside my area of expertise but I'm ~95% confident that it's wrong.
(I think there's a decent chance that you're correct, but that I've totally misunderstood this post, and the thing you're correct about is not accurately described by my summary.)
It sounds like you have a better understanding of cellular biology than I do, but I'm not impressed by mechanistic hypotheses about why some diet might be bad for your cells. Cells contain many mechanisms and it's too easy to come up with just-so stories about why some particular mechanism supposedly dominates. And I thought the provided mechanistic evidence was weak.
I am more interested in research where you give people different kinds of food and see what happens. I believe the best relevant source is the Cochrane meta-analysis Reduction in saturated fat intake for cardiovascular disease. It found that replacing saturated fat generally improved health (although the effect wasn't that big). It did not directly study PUFA, but most of the studies included in the meta-analysis replaced saturated fat with PUFA, so I take this as good evidence that PUFA is, at minimum, not less healthy than saturated fat.
I also think Dynomight's Thoughts on seed oil is quite good and more readable.
Prospective cohort studies support the healthiness of seed oils / unhealthiness of saturated fats, the best cohort studies being the Nurses' Health Study and Health Professionals Follow-up Study. You can reasonably question the reliability of cohort studies but it's nonzero additional evidence that they agree with the Cochrane meta-analysis.
On a more meta note, this post follows a classic pattern of (a) ignoring mainstream science, (b) not doing a wide review of the literature, and (c) cherry-picking a small amount of research that makes your hypothesis look favorable. It also makes very confident claims based on a mostly-untested hypothesis. Even without understanding any of the details of the science, that sort of writing pattern is a strong indicator that the hypothesis is false.
The blog post you cited for The Swamp follows the same pattern.
ETA: I searched on Sigma Nutrition which is another resource I like, and they have an article specifically addressing the claim that seed oil causes oxidative stress. They reference two different literature reviews that both found that it doesn't.
Some ~tangential points on metabolic syndrome / metabolically healthy obesity:
people with metabolic syndrome -- i.e., some level of insulin resistance
That is straightforwardly not what metabolic syndrome means. Someone with metabolic syndrome is likely to have insulin resistance but it is not guaranteed, and it's not part of the definition of metabolic syndrome.
Relatedly,
two types of obesity are recognized among those who study fat cells: "metabolically healthy" obesity, in which fat cells are overproduced and fat is stored normally, and "metabolically unhealthy" obesity, in which the number of fat cells is unaltered, but the individual fat cells hypertrophy from the body's pathological attempts to store more fat.
Your provided citation does not support the definition you give (see "Defining MHO" in the full text). The authors give several different but related definitions involving metabolic syndrome or variants thereof; none of the paper's definitions match yours. I am not very familiar with this area but I don't know of any reason to believe that metabolically healthy obesity (MHO) has anything to do with the number of fat cells. Some evidence against your definition is that people with MHO are much more likely to develop metabolic syndrome in the near future than healthy-weight people (source), which seems mechanistically implausible if your definition is correct (it would require people with MHO to lose fat cells despite not losing weight). Also the fact that (to my understanding) doctors routinely diagnose metabolic syndrome or metabolically unhealthy obesity without ever measuring the contents of patients' fat cells.
I got this info about MHO from Red Pen Reviews' Everything Fat Loss expert review. Red Pen Reviews is a website where nutrition scientists review nutrition books for scientific accuracy, and I consider it a good and trustworthy source to learn about nutrition science.
That is straightforwardly not what metabolic syndrome means.
On your model -- not an absent third party's -- what does metabolic syndrome mean? We can even taboo it. I'm talking about the thing that happens when people get abdominal obesity and have sugar and fat backed up in their blood and tend to be insulin resistant. The post gestures at "some level of insulin resistance" because it's the most frequently present facet that common medical tests can reliably identify.
Your provided citation does not support the definition you give
They don't say what they have identified as the mechanism of "metabolically unhealthy obesity" in the definition section.
If you read on to "Fat Distribution and Adipose Tissue (Dys)Function", you will see:
It is now generally accepted that central body fat distribution and an impaired adipose tissue function are better predictors of obesity-related metabolic abnormalities than total fat mass per se. VAT and ectopic fat accumulation, inflammation, impaired adipose tissue expandability and adipogenesis, as well as hypertrophy and altered lipid metabolism of fat cells, are anatomic and functional derangement of adipose tissue contributing to metabolic diseases and increased CVD risk.
.
Some evidence against your definition is that people with MHO are much more likely to develop metabolic syndrome in the near future than healthy-weight people (source), which seems mechanistically implausible if your definition is correct (it would require people with MHO to lose fat cells despite not losing weight)
The fat deposition mechanism and rate of lipogenesis, not the number of fat cells.
Are you aware of the "Adipose Tissue Expandability Hypothesis"?
Some of this reads like you missed this part, so I'm re-quoting it just to make sure you saw it:
***This paper directly shows that 4-HNE, an immediate and exclusive product of polyunsaturated fatty acid metabolism, decreases the activity of the electron transport chain in mice. Although this has not been directly shown in worms, outward signs of the electron transport chain slowdown syndrome shown by Chang et al. [ including fat gain ] have also been demonstrated in C. elegans by Singh et al.
[ ^ quote from above post ^ ]
.
1. If an insulin-resistant person eats a diet high in both fat and carbs, they are likely to develop a condition known as "The Swamp" characterized by weight gain and mental problems.
"The Swamp" isn't a condition; it's a set of acute side effects experienced for the ~24-48 hours after eating a moderate amount of sugar and fat in the same window.
3. "The Swamp" is caused by seed oils.
I don't think I mean this the way you think I mean it? I'm claiming metabolic syndrome is caused by seed oils, who then suffer from "The Swamp" whether they eat more seed oils or not.
4. Specifically, seed oils are high in polyunsaturated fatty acids, which cause cellular oxidative stress, which makes some people develop "The Swamp" when they eat carbs.
No, the oxidative stress wears down their mitochondria, which then experience "The Swamp" when they eat carbs and any fat, whether the fat has polyunsaturated fatty acids in it or not.
5. We know #4 is true because it was hypothesized by Randle et al. in 1963, and a 2009 paper found it to be mechanistically plausible. [No supporting empirical evidence is given.]
Huh? Did we read the same paper?
Randle (146) demonstrated that impairment of glucose metabolism by fatty acid (or ketone body) oxidation was mediated by a short-term inhibition of several glycolytic steps, namely glucose transport and phosphorylation, 6-phosphofructo-1-kinase (PFK-1), and PDH.
.
6. We also know #4 is true because humans are evolved to eat a low rate of PUFAs, and now we eat a high rate.
No. We know both of:
Humans evolved to eat a low rate of PUFAs, and now we eat a high rate
PUFAs are much more prone to peroxidation than saturated or monounsaturated fats
and we see the pattern of metabolic syndrome [ not your #4, which I'm not claiming ] rising in areas that adopt PUFA-heavy diets.
There is a large body of cohort studies claiming PUFAs are great for you. None of it, to my knowledge, compares people who have been eating ancestral near-zero levels of PUFAs, to people who have been eating "industrial" levels. For that cohort study, I would point you to "the variance in obesity and heart disease rates between nations, especially as of several decades ago [ ~1995? ] when they weren't as integrated into the 'second-world' diet yet".
[ what seems to be a typical paper from the Sigma Nutrition review ]:
However, despite multiple lines of complementary and concordant evidence indicating that n-6 PUFA intake is cardioprotective, some authors claim that n-6 PUFA intake leads to pro-inflammatory and pro-oxidative states (19–21), which is contributing to a growing social media movement against the use of vegetable oils (also called seed oils) (22,23). The goal of this narrative review is to provide health professionals, especially dietitians and other clinicians, with the information they need to address any concerns their patients/clients may have about oils containing predominantly UFA, including MUFA and PUFA.
So it's a non-epistemic paper, like those papers that [ openly declare that they exist to get more women on birth control ]
However, the results of most RCT show no effect of n-6 PUFA on markers of oxidative stress, including oxidised LDL(38) and F-2 isoprostanes, a marker of lipid peroxidation(39). As reviewed by Birben et al.(113), aerobic organisms have integrated antioxidant systems, which include enzymatic and non-enzymatic antioxidants that are effective in blocking harmful effects of reactive oxygen species.
No vertebrate and especially not large vertebrates has an antioxidant system comprehensive enough to prevent OXPHOS collapse from cumulative oxidative stress from being the major driver of aging.
It sounds like my summary was somewhat inaccurate but I basically understood your thesis. To avoid tangents, I will keep my reply focused on your central thesis.
First:
I said that RCTs (the ones where you feed PUFA to humans) have generally found that PUFA does not increase oxidative stress. This seems like strong evidence against your thesis. Do you disagree with my representation of what the RCTs have found? Or do you disagree that it is evidence against your thesis?
Second:
There is a large body of cohort studies claiming PUFAs are great for you. None of it, to my knowledge, compares people who have been eating ancestral near-zero levels of PUFAs, to people who have been eating "industrial" levels.
Okay, it sounds like you're claiming that the RCTs where PUFA looked healthy are invalid, because the people in the non-PUFA groups had previously eaten a lot of PUFA, so the damage had already been done, and the additional PUFA in the PUFA group didn't make them visibly worse off. Is that correct?
So if there was an RCT where group A ate minimal PUFA starting from birth, and group B ate minimal saturated fat from birth (+ a normal amount of PUFA), then according to you, group A would be healthier as adults. Is that correct?
(In case it's not obvious, I would bet on group B turning out healthier.)
If that's your position, then sure, RCTs and cohort studies can't falsify that position. But like, it seems implausible that there would be no detectable harms from eating PUFA? (And even more implausible that PUFAs would look healthier than saturated fat in the Cochrane review?) By analogy, if you had an RCT of current smokers and you randomly assigned half of them to quit smoking, I'd expect the quitters to have better health outcomes, even though the smoking has already done permanent damage to their lungs.
For that cohort study, I would point you to "the variance in obesity and heart disease rates between nations, especially as of several decades ago [ ~1995? ] when they weren't as integrated into the 'second-world' diet yet".
I am not aware of any peer-reviewed research on this, but Dynomight's article did look at country-level data on PUFA and obesity:
https://dynomight.net/seed-oil/#omega-6-doesnt-explain-inter-country-obesity
They found that
It seems to me that the observation "richer countries have more obesity" is better explained by the hypothesis "richer people can afford to buy more food".
The PUFA data are from 2010. If you think 2010 data is invalid, Dynomight's sources also included 1990 data, so you could look at that yourself if you want. (I'm not going to look at it.)
For the record, I don't find country-level correlation data very convincing (there are too many ways it can be confounded). I'm only reporting this because it's the evidence you suggested I should look at.
Third:
Regarding Chang et al. (2020) and Hue & Taegtmeyer (2009), I just don't consider cellular mechanisms to be compelling evidence. Especially cellular mechanisms in mice. I don't think this is an easy position to argue for or against, and I don't want to take the time to write a whole dissertation on why I believe this, so I will just leave my position un-defended.
I said that RCTs (the ones where you feed PUFA to humans) have generally found that PUFA does not increase oxidative stress. This seems like strong evidence against your thesis. Do you disagree with my representation of what the RCTs have found? Or do you disagree that it is evidence against your thesis?
Maybe the acute damage from PUFAs/HNE is too weak for some tests to detect; the load-bearing part for my theory is the cumulative damage. Nondetection of acute damage is weak evidence against cumulative damage.
So if there was an RCT where group A ate minimal PUFA starting from birth, and group B ate minimal saturated fat from birth (+ a normal amount of PUFA), then according to you, group A would be healthier as adults. Is that correct?
(In case it's not obvious, I would bet on group B turning out healthier.)
Yes, an RCT where some people were raised on infancy with a very-low-PUFA diet and still developed metabolic syndrome would disprove my theory. Props to you for being willing to explicitly bet on group B having better health outcomes.
[Dynomight] found that
There was no clear relationship between PUFA consumption and obesity. There was a positive relationship between GDP per capita and obesity.
I don't know how you get, from reading this article, "Dynomight concluded that there was no clear relationship between PUFA consumption and obesity". The scatterplots for [ PUFA consumption x obesity ] and [ GDP x obesity ] look pretty similar.
For the record, I don't find country-level correlation data very convincing (there are too many ways it can be confounded). I'm only reporting this because it's the evidence you suggested I should look at
I agree, country-level correlation data can be confounded by many factors. For instance, Korea has doctored their statistics to look like they have a much higher rate of obesity than they actually have. That's why I suggested looking at the variance, yourself, directly [ using "anecdata"/"intuition" ], instead of trying to find a reliable authority.
Sorry, looking back, I see I botched this in my last reply. I said "obesity and heart disease"; I should have said "metabolic syndrome and heart disease". I expect country-level correlation data on PUFAs vs obesity, specifically, to be partially drowned out by the fact that I don't think PUFAs cause more than 60% of obesity. I would look for correlation data on PUFA consumption vs rates of metabolic syndrome specifically, because I do think PUFAs cause more than 90% of metabolic syndrome and heart disease [ which I model as being caused by a slightly different mechanism that still shares the same source ].
Regarding Chang et al. (2020) and Hue & Taegtmeyer (2009), I just don't consider cellular mechanisms to be compelling evidence. Especially cellular mechanisms in mice. I don't think this is an easy position to argue for or against, and I don't want to take the time to write a whole dissertation on why I believe this, so I will just leave my position un-defended.
Did you see Singh et al., 2008 [ second link in the above quote ]? They're looking at C. elegans.
Insulins which humans inject are modified versions of cow insulin. Cows probably don't eat a lot of fat or carbs, right? They eat grass? I feel like someone should check what happens when you feed a cow the diet which puts humans who are taking insulin injections in the 'the swamp' state.
Grass is mostly (water and) carbs, just not carbs a person can digest and burn with any efficiency.
Presumably a Type I diabetic wouldn't experience "the swamp"? . . . I don't actually know, but I presume not?
Cows are actually the only commonly-eaten food animal which is relatively unaffected by a diet heavy in polyunsaturated fatty acids [ which cows in CAFOs, though not true lifelong "grass-fed beef" are indeed fed ]. Being ruminants, they fully break down any dietary fats before storing them in their bodies. Or at least this is the standard line peddled by the beef industry.
I Googled "do cows get diabetes", and apparently it's not really a thing except during long periods of lactation for dairy cows?
T1 diabetic here
What you call "The swamp" is one half well known to me
I cannot attest to any weight gain, as it's near impossible for me to "just gain weight".
The key I believe is that a definite craving for high-fat high-sugar food appears when the blood sugars are high, in quite a paradoxical fashion.
If I eat too much carbs and forget to take enough insulin for it, my BG can go from by preferred 4-5 to around 10 or above.
High blood sugars cause tiredness , confusion and exhaustion, clogged sinuses, dehydration, lack of joy, plus junk food cravings. Having "sweet piss" levels of blood glucose (>10 mmol) is comparable to a heavy food coma to me.
I can also compare it to being stoned from marijuana, minus all the good parts. Tired, lazy, confused, sleepy, frustrated, craving sweets.
>15 sucks as much as having a cold.
The evil thing is, people get acclimated to it (I did once too) and without experiencing it, the healthy reference loses it's importance.
I only know the layman explanation, but it's like any extra sugar "chokes" the metabolism, tissues are drowning in fuel while also starve, as insufficient nutrients reach them which leads to the cravings.
I feel this explanation is wrong, as I get mostly the same cravings regardless of insulin present in the blood.
I must stress the importance of those cravings, as it really is a bizarre upside-down reaction. It can make me stuff myself literally sick, "I must not move around one bit or it'll leak out" retarded full, waiting for a little more.
I never been anywhere close near obese my entire life, but I completely get the struggles of those people who must live like that, always tired and always hungry with no tool to solve it.
It's really not just lazyness, it's one devious fucking trap.
Another interesting tidbit is that I also require -much- less sleep (5-6h total) with borderline low blood sugars (3.5-4 mmol). Messing up dinner and having >10 sugars for the whole night makes me wake up as tired as I went to bed, even after 10 hours.
But the difference between all night 7 or 4 is enormous.
Also composing this reply fell apart a bit because I got distracted into it and forgot my dinner insulin, lol.
I must stress the importance of those cravings, as it really is a bizarre upside-down reaction. It can make me stuff myself literally sick, "I must not move around one bit or it'll leak out" retarded full, waiting for a little more.
. . . Huh.
I'm not a diabetic [ that I know of ], but I have this too. [ And I've never actually read anyone else describe something similar. ] It happens under conditions of prolonged mental stress. [ Usually but not always ] first my circadian rhythm goes, and get some amount of insomnia; at worst I stop being able to sleep more than 3-4 hours at a time. Then I start eating ridiculous amounts of calories, like 3,500/day when my RMR is 2,000 [ I end up having to or my brain basically won't let me sleep or move around and do stuff ]. During these periods, I experience some of the same mental effects you're describing, but as far as I know it's not directly blood sugar. I'd been hypothesizing it was something to do with the orexinergic system, since that has to do with both feeding and wakefulness, but I don't know.
Also, it's not exactly the same as yours, since at healthy baseline when I'm able to sleep more [ and thus my body is less "screaming for sleep" ], I also am less hungry. And when the sleepiness/insomnia/ridiculous-hunger-level thing is happening, I do gain weight.
I feel this explanation is wrong, as I get mostly the same cravings regardless of insulin present in the blood.
I'm a bit confused. You only get the bad cravings when your blood sugar should be spiking, right?
I.
It's a Known Thing in the keto-sphere [ I get the sense that r/saturatedfat is an example of this culture ] that people with metabolic syndrome -- i.e., some level of insulin resistance -- can handle fat or carbs [ e.g. either a ketogenic diet or something like the potato diet ] but can't handle both at the same time without suffering two symptoms:
[ 1 ] gaining weight
and
[ 2 ] suffering fatigue.
This is sometimes called "The Swamp".*
This is a very peculiar way for human metabolism to work. What's more, it only works this way for some people -- centrally, people who have acquired some level of insulin resistance [ "metabolic syndrome" ].
The insulin-resistant population leans not-young and slightly male, and its incidence is only appreciable in locations that have adopted a "Western diet".
The clearest articulation I've ever read of the Taubesian model of insulin resistance is actually a glowfic reply by Swimmer963:
This model has problems.
The most obvious is, "Why weren't the rates of metabolic syndrome high in poor agricultural communities that had to eat almost entirely carbs, then?" The American Heart Association's decision to blame the increasing quantity of fat in the 20th-century American diet for the increased incidence of "diseases of civilization" was, in a sense, perfectly natural, given that . . . that was the change in the American diet that had recently occurred! It's called the "nutrition transition": poor agricultural nations' consumption of carbohydrates decreases significantly when they come into some wealth! Yet their rates of metabolic syndrome generally go up [ if few are quite as high as the American one yet ].
A less obvious problem is -- ancestral humans didn't evolve to eat quite as many carbohydrates as even the modern American currently consumes, no. But they ate Swimmer's "one sugary meal" whenever they found a good payload of fruit! Why is the putative Taubesian pancreas "groan"ing?
A third problem with the Taubesian model of insulin resistance, that you would only notice if you frequently talked to people who get large health benefits from a ketogenic diet, is, it doesn't explain "The Swamp" -- the weight-gain-and-fatigue syndrome that occurs only when people on the insulin-resistance spectrum are eating a diet moderately high in both carbs and fat at the same time, and not when they eat a diet high in fat alone, or a diet high in carbs alone. If carbs are causing the syndrome, then why does eating only carbs alleviate it?
II.
One going hypothesis for what's causing "the Swamp" in those with metabolic syndrome is "the Randle Cycle".
Basically, there are a bunch of factors available in your cells [ such as malonyl-CoA ] which facilitate the metabolism of sugar and the synthesis of fat [ triglycerides ] for storage, which also marginally block the metabolism of fat, and a bunch of reciprocal factors which enable or are correlated with the breakdown of fat, and which marginally block the metabolism of sugar [ glycolysis ].
The theory says that when insulin-resistant people eat an appreciable amount of fat and an appreciable amount of carbs in the same ~24-h period, a self-reinforcing metabolic death spiral occurs between the "fat side" of the "Randle Cycle" and the "sugar side".
The "fat side" factors, activated by the influx of fat, block the breakdown of sugar. Simultaneously, the "sugar side" factors, activated by the influx of sugar, at once throttle the breakdown of fat for energy and facilitate its synthesis and storage.
Well, now there's lots of sugar backed up in the bloodstream, unable to be metabolized [ and probably exhausting the pancreas's ability to produce insulin ]. That sugar is going to keep pressuring the "sugar side" factors, which will block the metabolism of fat even harder. Simultaneously, the fat backed up in the bloodstream [ and, at the same time, the pathological vesicles of triglycerides building up outside the cell from the continued shouting of the "sugar side" factors to store more fat ] will keep pressuring the "fat side" factors, which will continue to block the metabolism of sugar . . .
But, okay, so there's a self-reinforcing metabolic death spiral. The "Randle Cycle" mechanisms for reciprocal inhibition of fat and carbohydrate metabolism must be universally conserved across humans. So why can some people handle a diet simultaneously heavy in fat and carbs for long periods of time without becoming obese, chronically fatigued, and insulin-resistant, while others can't?
The ancestral diet was moderately heavy in both fat and carbs [ yes, they ate lots of protein, but the Inuit invented the term "rabbit starvation" for a reason -- if you only eat protein, your body eventually stops being able to extract any calories out of it at all ]. So surely there's something additionally taxing about the diet of people who develop metabolic syndrome, beyond just "moderately high in both fat and carbs".
What changed, to spike the rate so wildly between ~1940 and ~1990, in "the West"?
III.
-- Aubrey de Grey, The Mitochondrial Free Radical Theory of Aging
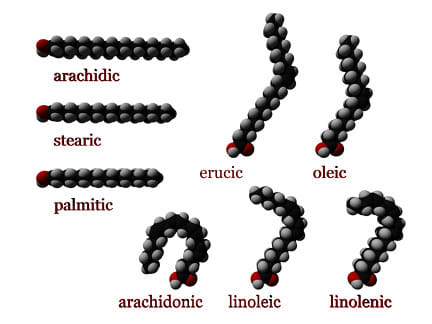
[ source: Wikipedia ]
Different fats are shaped differently.
Saturated fats are shaped like a straight line.
Monounsaturated fats are shaped like a kinked pipe.
Polyunsaturated fats, like the ones in "seed oils" -- rapeseed/canola oil, soybean oil, palm oil, etc., with the notable exclusion of olive oil [ for the most part ] and coconut oil -- are shaped like a U, and as de Grey says, have multiple C=C double bonds, which are very volatile when your mitochondrion attempts to metabolize them.
It's usually best for animals to use saturated fats, because they're the least volatile -- but if you're a cold-water fish or you need extra fluidity in your cell membranes for some other reason -- or if you're a seed and you want to be light so you can spread and you don't really care about volatility -- then lots of [poly]unsaturated fats will help, because the molecule takes up more space for the same number of atoms.
What would happen if you evolved to eat a low rate of polyunsaturated fatty acids, but instead your body found itself consuming a very high rate?
IV.
-- de Grey, The Mitochondrial Free Radical Theory of Aging
Your body's rate of "oxidative stress" from free radicals produced by oxidative phosphorylation in the electron transport chain, would go way up. This would damage your mitochondria. And damaged mitochondria damage mitochondria. The efficiency with which your body was able to do OXPHOS would decline, in a vicious cycle.*** Markers of oxidative stress would correspondingly go up in all your tissues.
With your body's core metabolic rate reduced, the Randle Cycle mechanisms might appear to become "antsier". A slight lag in the readiness of fat to flush from the bloodstream through the mitochondria would press harder on the "fat side" signaling pathway, while a slight lag in the readiness of sugar to flush from the bloodstream pressed harder on the "sugar side" pathway. Each side would throttle the metabolism of the macro triggering the other, bringing the overall metabolic rate even lower and exacerbating the process until the person had a chronic excess of sugar in the bloodstream, a chronic excess of triglycerides in the fat cells****, and chronic fatigue.
*Aspden describes a mechanism by which PUFAs inhibit glycolysis by downregulating mTOR/LKB1; the usual Randle Cycle mechanism for blocking glycolysis is that a high ratio of acetyl-CoA to malonyl-CoA will inhibit pyruvate dehydrogenase, but I thought the finding that PUFAs could block glycolysis even more strongly [ as I understand it, usually the Randle Cycle can't override mTOR, although the "fat side" "wants to" ] was illustrative, though not load-bearing.
**de Grey later goes on to say that he disagrees with the vicious cycle theory of mitochondrial aging, but this is only true in a very narrow technical sense; he doesn't dispute that the rate of damage to OXPHOS rises exponentially over time in a vicious cycle, he just thinks it's important to recognize that in "healthy aging", the rate of OXPHOS collapse is very uneven between cells.
***This paper directly shows that 4-HNE, an immediate and exclusive product of polyunsaturated fatty acid metabolism, decreases the activity of the electron transport chain in mice. Although this has not been directly shown in worms, outward signs of the electron transport chain slowdown syndrome shown by Chang et al. [ including fat gain ] have also been demonstrated in C. elegans by Singh et al.
****indeed, two types of obesity are recognized among those who study fat cells: "metabolically healthy" obesity, in which fat cells are overproduced and fat is stored normally, and "metabolically unhealthy" obesity, in which the number of fat cells is unaltered, but the individual fat cells hypertrophy from the body's pathological attempts to store more fat.
Appendix: Ancient Indian Seed Oil Consumers: The First Diabetics?
[ source [podcast] ]
[ Goodrich also claims the season of high monongo nut consumption gives the !Kung pot bellies and metabolic syndrome, because of the monongo nut's high linoleic acid levels, but that the low availability of calories is protective; I imagine either or both of "the monongo nut's LA content isn't actually 'Western'-level", or "these were !Kung who were also eating other things", is true, because as far as I understand it, the !Kung at the time they were studied by Western anthropologists in the late 20th century, were getting enough calories, and I doubt a diet of monongo nuts, which seem pretty normal as far as ancestral "reserve foods" go, genuinely gives you diabetes. ]
[ source ]
[ Wikipedia ]
[ as far as I know, all these cultures had some incidence of seed oil consumption -- sesame oil in the case of the Ayurvedics and rapeseed oil in the case of the Chinese ]