Also, I have no idea what this means:
"I think we are in the pre-theory stage for cancer. We are able to make progress against some forms of cancer, as we reduced lung cancer by public health efforts against smoking. But we don’t, to my knowledge, have the fundamental theory that we need, and so overall progress is slow."
We have TONS of data-backed theory about cancer from the level of evolutionary theory to the level of the mechanisms by which specific cancers are generated and progress. We are able to re-engineer the immune system in order to tailor treatments to a specific patient's cancer. It just costs a lot right now because you have to extract, engineer, culture, and re-insert cells into their body which is not simple.
The thing that most importantly distinguishes cancer from infectious disease as an opponent is that it is the body's own cells. That makes many cancers exceptionally difficult to target without harming healthy cells, and it equips them with the ability to develop a lot of specialized tools to evade the immune system. They also get a head start in terms of already being inside of the patient's body, and potentially also having a background of "nearly cancerous" cells.
I'd be surprised if you didn't know most/all of this already, which only makes me more confused about where the "pre-theory stage" comment comes from.
We don't really understand why the immune system sometimes kills cancer in its early stages and at other times fails to kill it in the early stages.
Given that Jason Crawford previously wrote about transposons being more important in the aging process than commonly assumed, I would expect him to believe that they might also be more important for cancer than commonly assumed.
But I do agree that immunotherapy-based approaches are an existing paradigm that has the potential to actually solve many cancers.
Having theoretical gaps is not the same thing at all as being pre-theoretical, but I agree with you that there are definitely big gaps in our knowledge. I'm not confident that Jason knows what the "commonly assumed" role of transposons is among cancer biologists, I think he either needs to be more precise in his language here or quote a more reliable source for the views of this expert community or preferably both.
Thanks @ChristianKI, but I think you're confusing me with someone else? I don't know what transposons are and I haven't written about them.
Maybe johnswentworth, from this one?
https://www.lesswrong.com/posts/ui6mDLdqXkaXiDMJ5/core-pathways-of-aging#Transposons
Thanks. Maybe “pre-theory” is too strong? Maybe “crucial theoretical gaps” is more accurate? I would be interested to hear from experts on this.
If you think we have the basic theory of cancer, the epistemic equivalent of the germ theory, I would be curious to know, when was that established? The germ theory was established around the 1880s or so, and it took several decades for all the solutions I described to be put in place, so maybe by analogy we are in that phase of just seeking effective (and affordable) solutions.
I agree that a lot of the difficulty comes from the fact that you're fighting the body's own cells. There are analogies to infection here too. We got sanitation before antibiotics because it was easier to kill germs outside the body (which you can do with heat, acid, bleach, etc.) than to find something that would selectively kill them inside the body while leaving the patient unharmed. Similarly, we have many broad-spectrum antibiotics now but basically no broad-spectrum antivirals, and I suspect that has something to do with the fact that viruses enter the cell in order to reproduce, meaning you have to take the battle inside the cell, disrupting the virus's life processes without interfering with the cell's. The cancer story is like a continuation of this “targeting” difficulty.
The germ theory of disease is, most essentially, the theory that infectious diseases are caused by invasion of the patient's body by a pathogen. It is defined by the type of thing that is the root cause of the disease - in this case, a non-human cell, virus, or even a malformed protein.
The direct equivalent in cancer is the theory that the cancer is made from a human's own cells growing out of control. That's a universally accepted fact and it has been for a long time. Again, I know that you know this, so I'm just really unclear about why you're proposing that we don't have the equivalent of germ theory for cancer.
But let's elaborate, because I am in biomedical engineering and it might be that I'm acquiring a little bit of expert syndrome, assuming that facts that are obvious to anyone in the field are equally obvious to those who are not.
You are correct in saying that the difficulty with cancer is fundamentally one of targeting! The nastiest cancer cells have evolved a range of capabilities:
- They look like healthy cells, so the immune system doesn't want to destroy them
- They produce signaling proteins that tell the immune system to calm down, which we call an "antiinflammatory microenvironment"
- They evolve resistance to chemotherapies, so that a drug and dose that would kill the cancer will also prove so lethal to healthy human cells that there's no benefit to administering it anymore
- They spread to new locations throughout the body. Since drugs don't always work equally well in all tissues, they might find a safe haven when they metastasize
- They rearchitect tissue in ways that are compatible with their own growth and survival
- They weaken the patient's physical health and physically infiltrate crucial tissue, making it riskier to operate
Figuring out a way to either get a drug to the physical location of the cancer cells but not to the healthy cells, or finding a drug that will selectively damage cancer cells but not healthy cells, is a huge challenge. Since cancer cells are not only alive and capable of evolution, but thrive off the same bodily conditions that normal cells survive best on, it's much harder to destroy them than it is to destroy bacteria (a challenge which is also becoming harder with the evolution of antibiotic resistance).
Fortunately, we are in the midst of figuring out a range of solutions to overcome these targeting challenges. Here are some examples, several of which we're actively doing in my own lab:
- Cancer creates an acidic pH nearby, and we can devise drug delivery systems that are activated by low pH
- We can take out patient immune cells, equip them with genetically engineered detectors for the patient's specific cancer, and then re-introduce them into the host
- We can drive a pro-inflammatory state at the cancer site so that the host immune cells can eliminate the cancer instead of getting shut down when they're nearby
- We can destroy all the cancer cells with really severe treatments that would normally kill the patient, but save and reintroduce the cells needed to allow the host to regenerate and survive after the treatment
- Some cancers are caused by infectious diseases, and we can vaccinate against those
- We can do genetic testing for things like breast cancer-promoting genes, and women can choose to have a preventative masectomy to reduce their risk from these genes. That's just one well-known example, other non-vital organs can be removed from both men and women as needed if they are at risk of developing cancer.
- We can carefully break down the architecture of the cancer to figure out how it grows and develops, then carefully interrupt crucial links in that process in ways specific to individual cancers
- We can identify and eliminate mutation-causing carcinogens from our environment
- We can engineer synthetic or cell-based replacement organs, so that if excising the cancer would normally require destroying a critical organ, we can just take it out and then replace it with a new organ that doesn't have any cancer cells in it
- We can create sensors for cancer that detect it at an earlier stage when it's easier to treat
- We can look for things besides just cell division that the cancer needs to do more than normal, like growing new blood vessels, and inhibit those activities
- We have an ever-expanding list of ways to target cell growth and division, which cancers do more than normal human cells (but which human cells also do, which is why you lose your fast-growing hair during chemo).
This is by no means an exhaustive list, cancer isn't even my research topic, this is just stuff I've picked up from my labmates who work directly on cancer research.
The reason we have so many different strategies for tackling cancer, all tractable, is that we understand cancer really in quite a lot of detail, such that even though it's a really difficult collection of illnesses to treat due to being made of our own cells running amok, we are still able to deal with many cancers rather successfully.
There are other diseases of old age for which I'd be much more comfortable with the statement that we have no established, consensus basic understanding of how the disease works. Alzheimer's is one. Aging itself is by no means pre-theoretical, we have many decades of evolutionary theory to explain it in principle and nowadays a lot of specific biological mechanisms to flesh out the picture, so to speak.
Cancer is a case where the reason we haven't cured it, despite decades of intense research, is that it's a really tough problem and we've only developed the kinds of sophisticated tools that might let us bring the hammer down on it in the last couple decades. As you know, FDA trials take a long time, and so it takes a long time to move from basic research to the clinic.
You can see our progress though at the CDC, which shows a steadily decreasing death rate from cancer over the last quarter century.
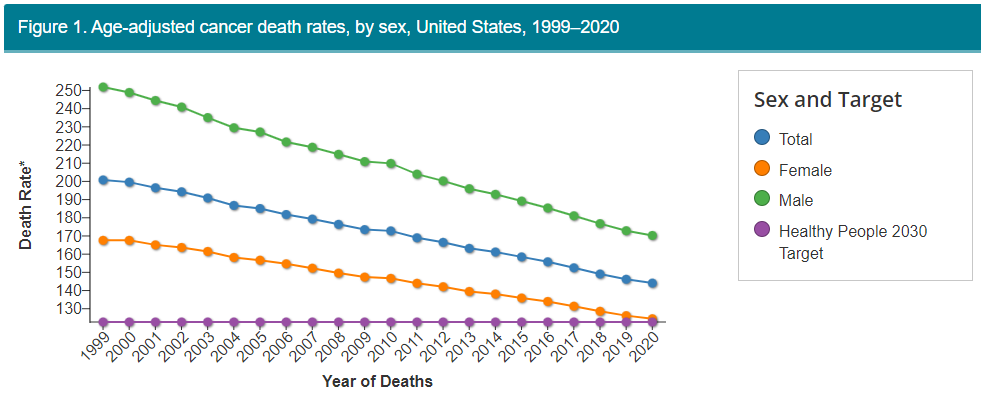
And remember that not all of the deaths that do occur are failures of medicine. Some are because patients do the unhealthy stuff we know gives them cancer anyway, despite warnings, or they're not proactive about checkups, or they don't have access to care, or they refuse or can't afford treatment.
Meanwhile, we are continuing to build up our technology stack, finding ways to do things like safer and more robust gene therapy. Remember that CRISPR for all its hype is like a decade old discovery, so drugs that exploit the refinements we've made to it since then (which are considerable) have really only had a few years maybe to get tested in preclinical models.
Whatever medications are coming out today are the biomedical equivalent of ancient light from distant stars, showing us the picture of what biomedicine was capable of 15 years ago. In a regime of explosive exponential growth in technology, particularly biotech, there's going to be a huge gap between the insane stuff we can engineer in the lab today and what patients are able to take advantage of in the hospital. That might look like some sort of stagnation, but it's not, it's just the nature of the beast when human lives are on the line if you fuck up the technology and the rules aren't built from the ground up with math the way they are on computers. Personally, I'm thrilled with the biomedical universe and it feels like it's moving insanely fast, but I understand why it might not look that way if you're not working in the field as a professional because of the time delay between discovery and drug.
You've made a bunch of great comments in this thread. Have you considered turning them into a top-level post on LW and/or the EA forum? You've already done the laborious part of writing all this stuff up, after all. From my perspective, the only things missing to turn them into a post would be to add a bunch of headings, plus maybe an intro paragraph, and to address a general audience rather than jasoncrawford specifically.
This is great, thanks. I added a link to this comment in the body of the post.
Where I was coming from was:
- We have put a lot of resources into fighting cancer:
- We declared a “War on Cancer” ~50 years ago
- There are over $7B for it in this year's appropriations act, about 15% of NIH's total budget
- There are also lots of private foundations working on it
- It is the canonical example of a big, important thing to be working on
- We seem to be making only slow progress
- It is still the number one cause of death
- Scott Alexander's summary was “gradual improvement”
- Our treatments (surgery, radiation, chemo) seem crude and/or to have horrible side effects; some even people amputate body parts in order to survive
- Some cancers are still not detected until very late
- It seems like there are lots of open questions about how cancer works (general impression)
I added that up and thought “well I guess we just don't understand it well enough yet.”
I think you've convinced me that “pre-theory” is wrong. But I don't think you can explain slow progress just by saying “this is a hard problem.” Infectious disease was also a hard problem! Nuclear physics was a hard problem. Etc. And we have way more resources to devote to the problem now (fact-check/citation needed, but without yet researching it I'm ~80% certain of this).
Based on the data in your chart, 21st-century cancer progress looks to be roughly half the speed of 20th-century infectious disease progress. So now I'm wondering, what would it take to double the speed of cancer progress? Do we need a breakthrough in science, in our understanding of how cancer works—filling in a gap in the theory? Or do we just need a breakthrough in technology? Or did the breakthrough already happen and it just needs to be made cheaper, or something like that?
At this point, we’re leaving the land of empirical fact behind and entering the conjectural realm.
With that caveat, I’m going to give two answers: cancer really is harder than infectious disease, and we are still mainly in a paradigm of treating diseases rather than fighting aging.
With infectious disease, we have two powerful strategies that are lacking in cancer. One is targeting the radically different physiology of infectious agents. Here, the targeting problem that impairs cancer therapies is much reduced. We had antibiotics and vaccines long before we had effective chemotherapies in large part for that reason.
Second is targeting the radically different life cycle of infectious agents. Besides STIs, infectious agents have to pass through an external environment to transmit between hosts, and that gives us an opportunity to intervene. We can purify water, cook food, socially distance from the sick, and exterminate vectors like mosquitos. Cancer originates within you, so we just can’t use this strategy.
I’m no physicist, so I can only gesture to a couple structural factors there. One is that with physics, you can directly test your hypothesis on a machine you build from the ground up, whereas in biology, you have to do all your research in an organism that wasn’t designed to accord with theory, and where there are enormous ethical barriers to just testing your ideas directly. You can’t just give somebody cancer and see if your chemo drug helps.
So point A is that a range of specific factors make cancer especially tough to solve relative to infectious disease or nuclear physics. It’s a little like your post from a while back about “why wasn’t this invented earlier,” but in reverse. We can point to specific factors, the ones I’ve just made, that are strong reasons to explain why it has taken longer to bring cancer deaths way down than infectious disease deaths.
Point B is that even now, we focus a lot on specific diseases like cancer that are actively causing patients suffering and are the most legible immediate causes of death. The whole anti-aging field starts by saying “by the time you’ve got old age diseases like cancer, your body’s systems for maintaining itself have gotten seriously impaired. Maybe we can slow or reverse that aging process so that instead of treating dangerous cancers in a body that is prone to getting another cancer soon after due to its advanced age, we just have bodies far less prone to cancer.”
Personally I think that sounds very promising and also has plenty of theory and data, and it’s where I plan to steer my research toward, but it’s also a field with no proven successes yet at least from self-described “anti aging” research. There are pre clinical trials underway, such as a trial of low dose rapamycin in dogs to establish safety and efficacy for as an anti aging drug in a species with similar physiology that shares our environment.
But again, echoes of ancient light: the anti aging field was barely a thing 20 years ago, so we’re seeing those early finds from Lab Centauri just arriving on Planet Clinic now. Last year tons of money poured into the field and it’s way more visible now, so if it’s not just a hype train we might see some truly revolutionary stuff around 2040.
The closest thing I can think of to, if not pre theory then “paradigm shift” in cancer is a refocusing of effort on slowing and reversing aging rather than treating cancer after the body is already in bad shape from a lifetime of biochemical warping.
If you want to double the speed of cancer progress, you’d need to shorten the time it takes to go from lab to trial to clinic without compromising patient safety and willingness to participate in trials. Also just keep dumping money in the space, although cancer probably isn’t your best bang for buck option as far as saving lives with biomedicine.
This isn’t cancer, but the Kidney Project has made a lot of progress on bioartificial kidneys and they tell me they need $10 mil to get through human trials. But it’s hard to come by the funding. So dump $10 mil on them and maybe you’ll cure kidney disease while reducing or eliminating a horrific organ black market.
As far as I know (I didn't work in cancer research but did get a masters in a related field) all cancers have an active mutated gene. This means there is something detectable in every cell we consider part of the tumor : an mRNA with the mutated sequence.
I don't know of a biological mechanism where cancer could be possible without this.
So scientifically speaking, we are post theory, and there are not important theoretical gaps.
Why aren't there treatments based on this? Well there are. In rats...
I'm not sure if most biologists would in fact say that you have to "cure cancer" one disease at a time, especially if we define "cure" to encompass "prevent." As a trivial example, the CDC says smoking causes " cancers of the mouth and throat, voice box, esophagus, stomach, kidney, pancreas, liver, bladder, cervix, colon and rectum, and a type of leukemia (acute myeloid leukemia)." Quit smoking and you (help) cure like 10 different cancers.
Are you familiar with Aubrey de Grey's thinking on this?
To summarize, from memory, cancers can be broadly divided into two classes:
- about 85% of cancers rely on lengthening telomeres via telomerase
- the other 15% of cancers rely on some alternative lengthening of telomeres mechanism ("ALT")
The first, big class, can be solved if we can prevent cancers from using telomerase. In his 2007 book "Ending Aging", de Grey and his co-author Michael Rae wrote about "Whole-body interdiction of lengthening of telomeres" (WILT), which was about using gene therapy to remove telomerase genes in all somatic cells. Then, stem cell therapy could be used to produce new telomeres for those cases where the body usually legitimately uses telomerase.
But research has moved on since then, and this might not be necessary. One promising approach is Maia Biotech's THIO, a small molecule drug which is incorporated into the telomeres of cancer cells, compromises the telomeres' structure, and results in rapid cell death. They are currently preparing for phase 2 for a few different clinical trials.
For the other 15% of cancers, the ALT mechanism is as far as I know not as well understood, and I haven't heard of a promising general approach to cure it. But it seems plausible that it could be a similar affair in the end.
You should probably differentiate more between curing and preventing. Most of the infection gains were in preventive measures - both in avoiding infections and boosting the body's systems. And this is where massive anticancer gains have been possible (e.g. banning asbestos and dissuading smoking).
The low hanging gains in actual curing were generally mechanisms that make use of differences between different kingdoms, e.g. antibiotics targeting bacteria cell walls, since they have a different structure than eukaryotic cells. The problem with cancers is that they're your own cells that are going wild, for a whole host of reasons. This means that any successful anti-cancer thingy will either have to specifically target cancer cells (which is generally hard) or be area spells, that will also kill off healthy cells. Pretty much what DirectedEvolution wrote.
Good point, I say “cure” here but yes I really mean any combination of prevention + cure that solves the problem. You're right that prevention was the majority of the success against infection (and this may be true for cancer as well).
Just want to say that a lot of the conversations here are revolving around using our current model of molecular biology to treat cancer. That model has had a ton of success, and is the best workhorse we have for reliably turning money into life-years probably. I think it's a big reason why a lot of people throw that idea around that cancer is gonna be cured by just developing a super-cornucopia of drugs.
But I think the heart of this post is in asking "well, are there models that get OUT of that framework? ones that are seismic shifts, like the germ theory of disease?" Yeah, just the one(s) that I've seen are super theoretical. Michael Levin's group, oft heralded on LessWrong bio posts, is in fact starting to study cancer. The argument goes that, like Archimedes mentioned in the comments here, cancer is a disease of individual cells rebelling against multicellularity. Levin's group thinks that the mechanisms for that control might be electrical/physical — as in, the deformity is more in the "software" (transient signals dictating cell behavior) being run by the "hardware" of whatever mutated proteins are in the cell.
It's a cool idea, but the work that I've seen on that front is really preliminary. I think the most experimental work I've read by them is a paper that shows that brain cancer cells are killed by drugs which modulate voltage-gated channels. Ostensibly, this modulates some of the transient signalling on the electrically based "software level" which is driving them away from multicellularity. [I am personally extrapolating these claims.] But I think it's more likely that they're seeing a response b/c voltage-gated protein activity is hard-wired to cell survival mechanisms.
I listened an interview recently and found it an intriguing perspective on cancer:
https://80000hours.org/podcast/episodes/athena-aktipis-cancer-cooperation-apocalypse/
My oversimplifying summary is that cancer is essentially a multicellular alignment problem. I interpret this to imply that there’s no silver bullet to getting all your cells to behave in a fully cooperative way with the evolutionary pressures involved. I believe we can get better at encouraging cellular coordination but “curing” cancer entirely would likely take technology at roughly the level of nanobots intelligently and locally targeting cancer at the cellular/molecular level.
With lymphocytes, we already have a template for nanobots to do the job.
Human cells display fragments of proteins within them on their cell walls. Cancer means that cells have a lot of mutated proteins that they would present on their cell walls. Cancer call also shut down the process of protein fragments being shown on the cell walls but that's also detectable by lymphocytes.
There's no good way for a cancer to display all the fragments that it's supposed to display while at the same time not displaying any mutated protein fragments.
Our body does produce the nanobots. But naturally, our immune system isn't perfect at making the right nanobots. There are clinical attempts to grow the right nanobots in vitro and use them to attack cancer.
In vitro approaches could be improved on computer models that guide the process.
We need one machine learning model that you give a DNA sequence of a cell and that then tells you what protein fragments that cell will display.
We need another machine learning model to tell us how the lympocytes need to be programmed to match recognize a cell that displays those fragments. It's a simulation task that's a bit more complex than what alpha fold is doing.
And then we need a good process to grow those lymphocytes in a cost-efficient manner. Likely, something where you synthesise DNA and create a system where that DNA gets used.
The other way to do it is a protein based mechanism that you inject into each cell. If a specific mRNA sequence matches to the mechanism, it causes the cell to self destruct.
Package the mechanism in a virus and inject the virions directly into each tumor. This would cause the cancerous cells to die and the healthy ones to usually survive. (Just injecting a bunch of junk into a healthy cell can make it fail)
Advantage here is it is a general solution. All cancerous cells have an mRNA that is mutated. You can build a different matching sequence for any cancer. So it is a "cure", there will never be a cancer you can't treat.
If you could also grow replacement organs - since this battle may scar the patients existing ones and cause other damage - and everything was done by AI systems using high speed robotics that learn from each mistake across all patients (human doctors learn 1 patient at a time until they are forced to retire from aging, AI doctors can learn from a million attempts in parallel) - theoretically the cancer death rate would trend towards zero.
With that said each patient would probably have to live maybe for years in a sealed biolab, surrounded by racks of equipment and plumbing for their external organ support systems etc. It's easy to imagine a system that would keep someone alive indefinitely if their body is spread apart across a large life support system. It's harder to imagine putting them back together in a way that won't have high mortality rates once they leave from unobservable small mistakes.
Note by "easy" I mean with effective narrow AI. What keeps the patient alive for years when human facilities can't is each domain of the human body (liver, circulatory, hemopoietic, immune, nervous, etc) is monitored by multiple parallel narrow AI, and each piece of hardware has multiple parallel systems. So it's parallel redundancy: all the hardware for a domain have to fail at the same time and all narrow AIs have to fail to take a corrective action.
So for example for the patients blood chemistry to get outside the narrow ranged associated with long survival, each living artificial liver must fail at the same time, and all monitoring AI systems must fail to observe this, and other systems for related organs must fail also.
It can be made unlikely, where the probabilities work out that a patient on total life support has a longer expected lifespan than a healthy 12 year old human who didn't age.
In an excellent recent essay on “big visions for biology,” Sam Rodriques writes:
Are “most biologists” right about this?
We can get perspective on this from the history of infectious disease. After all, infection was also “many diseases,” with disparate causes (viruses, bacteria, protozoa) and disparate pathways (air, water, food, insects). And yet, while we did not exactly “cure infectious disease” (just ask the 248,544 people who got covid last week in the US alone), we did reduce every major metric of infectious disease by 90% to 99% in wealthy/developed countries:
Infectious disease was not the whole story in all of the above metrics, but it was the biggest factor in all of them.
We also eradicated smallpox worldwide and eliminated diseases such as cholera, malaria, and polio from many countries:
Did we have to cure all these diseases “one at a time”? No, not exactly:
Underlying all of this is a theory that explains the basic causal mechanism of infection: the germ theory. The theory alone doesn’t give us everything we need to cure each specific disease: new research is needed to understand the etiology and epidemiology of each one. But the theory does give us a conceptual framework and a set of tools to guide that research. Before the theory, we were able to make limited progress against some diseases; after it, we made much more rapid progress, and the diseases we weren’t able to solve became the exception rather than the rule.
I think we are in the pre-theory stage for cancer.We are able to make progress against some forms of cancer, as we reduced lung cancer by public health efforts against smoking. But we don’t, to my knowledge, have the fundamental theory that we need, and so overall progress is slow. [UPDATE: @DirectedEvolution has convinced me that “pre-theory” is wrong, see their excellent comment below.]Infection and cancer are both “many diseases”—but those diseases have something in common. A deeper understanding of cancer will allow us to make much more rapid progress, on more fronts. There won’t be a silver bullet—no single treatment that will cure all cancers. More likely there will be a few major techniques, as with infection we had to develop sanitation, vaccination, and antibiotics; and even within each of those categories, we needed many specific efforts to develop each individual vaccine, antibiotic drug, and sanitation method.
But there is no reason why we can’t do for cancer what we did for infection: reduce it by orders of magnitude and knock it down from a number one cause of death to a much more minor and more manageable threat. And eventually, with more advanced science and technology, perhaps we will be able to truly cure both of them.